Introducing the Empirical Formula Worksheet and Answers, an authoritative resource designed to empower students and practitioners alike in the realm of chemistry. This comprehensive guide delves into the fundamental concepts of empirical formulas, providing a structured approach to understanding their significance and applications.
Through engaging worksheets, detailed answer keys, and thought-provoking discussion questions, this resource fosters a deep comprehension of empirical formula calculations. Real-world scenarios and interactive tools further enhance the learning experience, ensuring a thorough understanding of this essential chemical concept.
1. Empirical Formula Basics

An empirical formula represents the simplest whole-number ratio of elements in a compound. It provides crucial information about the compound’s composition, allowing chemists to understand its structure and properties.
Empirical formulas differ from molecular formulas, which indicate the exact number of atoms of each element in a molecule. Determining empirical formulas is essential for various chemical analyses, such as identifying unknown compounds and calculating molecular weights.
2. Worksheet Activities: Empirical Formula Worksheet And Answers
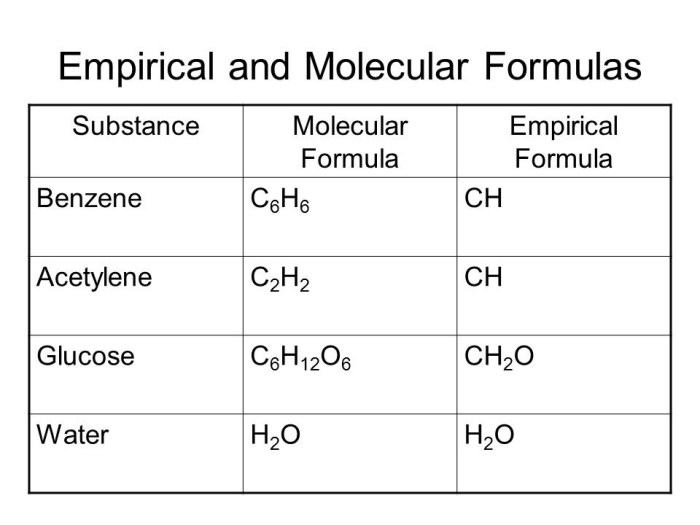
The worksheet comprises practice problems that guide students in calculating empirical formulas from diverse data sources. These problems include determining empirical formulas from percent composition, mass spectrometry results, and other relevant data.
By working through these problems, students develop a strong foundation in empirical formula calculations and gain experience applying them in different scenarios.
3. Answer Key
The answer key provides comprehensive solutions to the worksheet problems. Each solution includes step-by-step calculations and clear explanations of the concepts involved.
The detailed answers help students understand the underlying principles of empirical formula calculations and identify areas where they need further improvement.
4. Extended Exercises

The extended exercises challenge students to apply their understanding of empirical formulas in more complex scenarios. These exercises may involve real-world applications, case studies, or problems that require students to combine multiple concepts.
By completing these exercises, students develop a deeper understanding of the practical significance of empirical formulas and their limitations.
5. Interactive Table
The interactive HTML table allows users to input data, such as percent composition, and instantly calculate the corresponding empirical formula.
This tool provides a convenient and user-friendly way for students to practice empirical formula calculations and visualize the relationship between percent composition and empirical formula.
6. Discussion Questions
The discussion questions encourage students to reflect on the broader implications and limitations of empirical formulas.
- How do empirical formulas differ from molecular formulas, and what are the implications of this difference?
- Discuss the role of empirical formulas in chemical analysis and their limitations.
- Identify potential sources of error in empirical formula calculations and suggest strategies to minimize them.
These questions foster critical thinking and help students develop a nuanced understanding of empirical formulas.
FAQ Insights
What is the purpose of an empirical formula?
An empirical formula represents the simplest whole-number ratio of elements present in a compound, providing insight into its elemental composition.
How do I determine the empirical formula of a compound?
To determine the empirical formula, you need to know the mass or mole fractions of each element present in the compound and then simplify the ratios to the smallest whole numbers.
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest whole-number ratio of elements, while a molecular formula indicates the actual number of atoms of each element in a molecule.